For Lab Professionals
Capitainer® samples can be easily adopted in any laboratory.
This page provides an overview of laboratory handling procedures for Capitainer® cards, with a focus on establishing efficient workflows and supporting method development and validation activities.
The accompanying videos demonstrate pipetting onto Capitainer® cards under controlled laboratory conditions, as part of laboratory validation workflows.
For guidance on device use when applying blood obtained via fingerstick (capillary) collection, including laboratory use cases, please refer to the user focused instructions and capillary sampling documentation.
Our team of experts is available to support you throughout method development, validation, and implementation of Capitainer® devices within your laboratory workflow. Please contact us for personalized guidance tailored to your specific analytical needs.
1) Laboratory validation – Use of blood from tubes
Capitainer® devices are designed for use with fresh blood. However, during early validation or method development, it is often practical to begin with previously collected venous blood and apply it to the cards by pipetting under laboratory conditions.
In our development work, we typically start by using EDTAanticoagulated blood to generate samples for method development and optimization. Previously collected blood may be used with Capitainer® B10, Capitainer® B50, and Capitainer® SEP10.
When working with previously collected blood, please consider the following:
- Blood age and storage
Do not use blood that has been stored refrigerated for more than three days. Allow refrigerated blood to equilibrate to room temperature before application to the cards.
- Unsuitable sample types
Lyophilized blood products and previously frozen blood are not recommended and may not perform as intended
- Mixing prior to use
All blood samples must be brought to room temperature and thoroughly mixed on a rocker or equivalent device before application to ensure homogeneity.
- Timing after mixing
If using a syringe dispenser or pipette, apply the blood to the cards within 5 minutes of the final mixing step to minimize sedimentation.
- Drying
Allow the sample discs to dry overnight at minimum while remaining in the card. Wet or partially dried discs may not detach correctly from the card during downstream processing.
- Recommended application volumes
Use the following volumes and procedures to best mimic real world sampling conditions:
Capitainer® B10
Apply 25 µL as a single drop to each inlet, simulating blood application from a finger prick.
Capitainer® B10 Vanadate
Apply 25 µL as a single drop to each inlet, simulating blood application from a finger prick.
Capitainer® B50
Aspirate 60 µL of blood and fill the first channel to the stop line. Repeat for the second channel.
Capitainer® SEP10
Aspirate 60 µL of blood and fill the first channel to the stop line. Repeat for the second channel.
Note: It can take up to about 5 minutes for the blue color in the control window to appear.
Laboratory validation – Use of Urine
Capitainer®DIP70 is intended for use with fresh urine collected in a container, where the card is dipped into the sample. For method validation or situations with limited sample volume, a smaller volume may be applied directly to the sample discs using a pipette.
Capitainer® DIP70
Aspirate 100 µl urine and apply it on the first discs. Repeat for the second disc.
2) Pre-Analysis – Sample Disc Access and Removal
The sample disc in the blood cards is accessed from the reverse side by peeling away the protective tabs. For Capitainer® DIP70, the sample discs are accessible immediately after removal from the drying pouch.
Sample discs may be removed manually using tweezers and transferred to a suitable tube or plate for extraction. We recommend using flat tip tweezers for reliable handling.
A Capitainer provided disc removal tweezer is available for this purpose.


Automated and Semi Automated Handling Options
Capitainer also offers a semiautomated preanalytical handler, the PH96, which streamlines sample disc removal while ensuring full traceability of each sample throughout the downstream analytical workflow.
For higher throughput applications, Capitainer provides a fully automated solution, the PA496, which is capable of processing stacks of cards with minimal manual intervention.
In addition, BSD Robotics provides instruments compatible with Capitainer® cards.
Residual Paper Fibers
After sample disc removal, you may observe small traces of paper fibers remaining on the card. These fibers originate from the adhesive layer that secures the discs within the card.
This is a normal and expected observation. The amount and appearance of residual fibers are consistent and controlled, and this behavior has been fully accounted for during the design and development of the cards. The presence of these fibers does not affect sample integrity or downstream analytical performance.
3) Sample Reconstitution and Extraction – Transform back into liquid form
After sample disc removal, the sample is reconstituted into liquid form for downstream analysis. The selection of extraction buffer and elution protocol depends on the target analyte and the intended analytical method.
Optimization of dried blood sample extraction involves multiple variables, and established published methods for conventional dried blood spot (DBS) samples are generally applicable to Capitainer® samples and may be used as a starting point for method development.
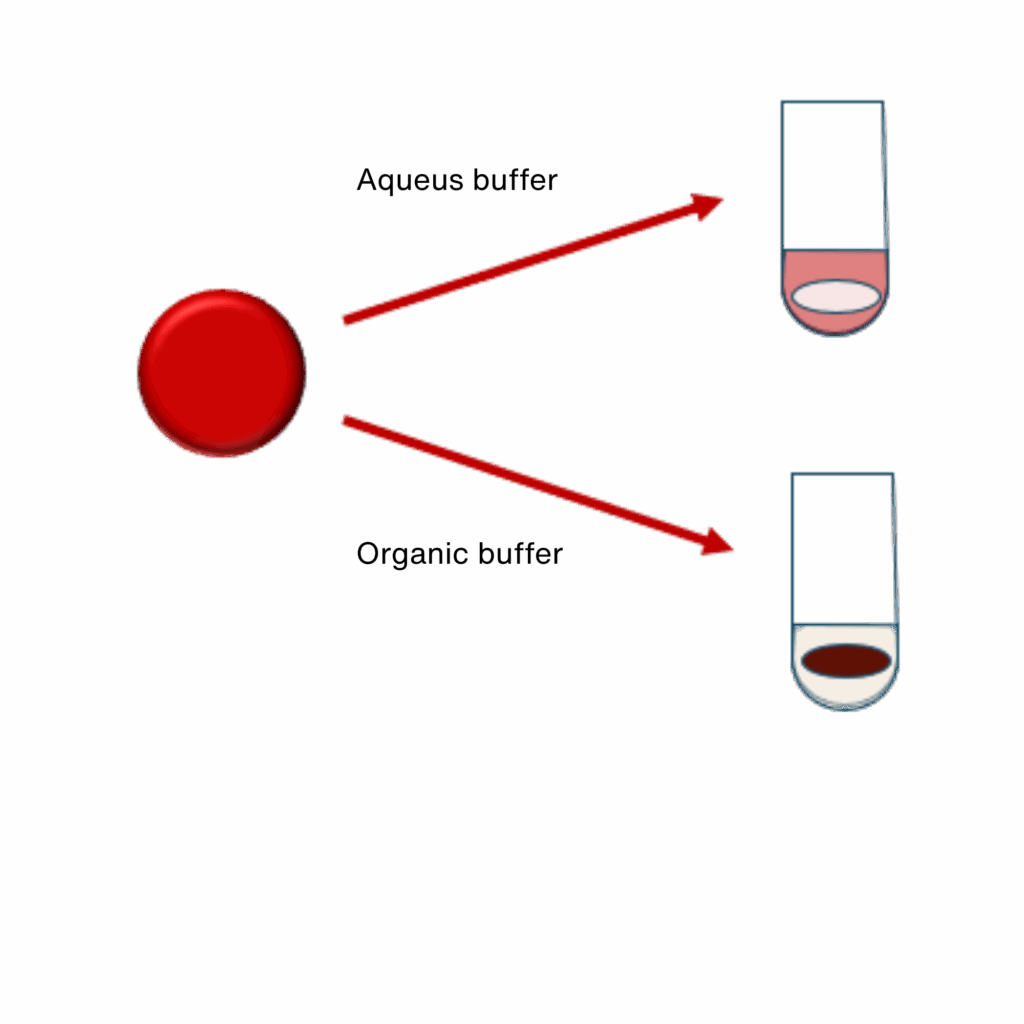
A key consideration is the choice between aqueous and organic solvent–based extraction:
- Aqueous extraction
Aqueous buffers are suitable for recovery of most analytes and are required for larger molecules, such as proteins. This approach results in a fully hemolyzed wholeblood extract, typically characterized by a red coloration due to the presence of hemoglobin. - Organic solvent extraction
Organic solvents cause protein denaturation and binding to the paper matrix, while metabolites and small molecules are released into a clear, protein depleted eluate. This approach is commonly used when targeting lowmolecularweight analytes.
The elution buffer volume is an important parameter in sample extraction. Higher elution volumes generally provide greater consistency and higher analyte recovery, but result in a more diluted sample, which may affect assay sensitivity.
As an initial starting point, we recommend the following elution volumes:
- 10 µL sample disc: 100–150 µL elution buffer
- 50 µL or 70 µL sample disc: 300–500 µL elution buffer
Please note that sample discs retain a small, inherent volume of liquid that is not recovered using standard elution procedures. This volume loss is intrinsic to paperbased sampling. The use of filter plates or filter tubes can help minimize unrecovered volume and improve overall extraction efficiency.
Extraction Using Standard Procedure:
Add the selected extraction buffer to the tubes or plate wells containing the sample discs. Vigorously agitate the tube or plate containing the disc and elution buffer for 30–60 minutes to facilitate analyte release.
After extraction, aspirate the eluate and transfer it to a clean tube or plate for downstream analysis. This step may be fully automated using commercially available liquid‑handling robots.
As an alternative, the sample disc may be removed from the tube or well instead of transferring the liquid.
When large elution volumes are used, the sample disc may remain at the bottom of the tube without interfering with liquid aspiration by the analytical instrument.
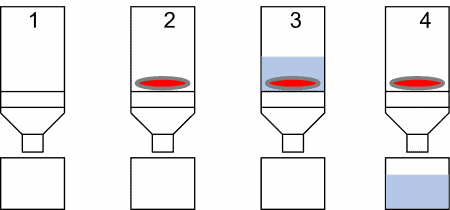
Extraction Using Spin Columns or Filter Plates:
To reduce elution volume and incorporate a sample cleanup step, extraction using spin columns or filter plates may be considered. This approach also enables recovery of most of the liquid absorbed by the sample disc, which is otherwise not retrieved during standard elution.
Approximate liquid retention by sample disc type is as follows:
- Capitainer® B10: ~20 µL
- Capitainer® B50 and Capitainer® DIP70: ~70 µL
General Procedure
- Select an appropriate filter type compatible with the target analyte and downstream analysis.
- Place the sample disc into the spin column or filter plate well.
- Add the extraction buffer and vigorously agitate for 30–60 minutes.
- Centrifuge to collect the eluate, then discard the column and sample disc.
Automation Considerations
For automated workflows, this extraction approach offers the advantage of producing an eluate free of residual sample discs in the wells. This allows liquidhandling robots to aspirate from lower volumes without obstruction.
Many commercially available liquidhandling systems are compatible with filter plates, enabling a fully automated and scalable preanalytical workflow.
4) Downstream Analysis and Result Interpretation
The eluted samples are ready for analysis on the selected analytical platform. Capitainer® samples have been successfully applied across a wide range of analytical techniques, including but not limited to:
- Clinical chemistry
- Immunochemistry
- ELISA
- Mass spectrometry
- PCR-based applications
Results generated from Capitainer® extracts represent analyte concentrations in the extract, which is typically not the native clinical reporting format. For laboratory developed (inhouse) tests, this is commonly addressed by incorporating appropriate calibration materials and standard curves to generate results in the desired reporting units.
When Capitainer® extracts are analyzed on instruments designed and calibrated for a different matrix, most commonly serum or plasma, the reported values will not directly correspond to the original sample matrix. In such cases, a correction factor must be applied.
This correction factor should account for:
- Matrix differences (whole blood vs. plasma or serum)
- Dilution introduced during extraction
- Extraction efficiency
The correction factor must be established, validated, and documented during method development and validation.
Traditional dried blood spot (DBS) cards have been used for more than 60 years, and while they do not provide the same level of accuracy and precision as Capitainer® solutions, the extensive body of analytical knowledge and published methodologies for dried samples may still serve as a useful reference during method development. The review ”State of the Science in Dried Blood Spots” [State of the Science in Dried Blood Spots | Clinical Chemistry | Oxford Academic] describes a wide range of analytes measurable in dried matrix samples.
5) Shipping – Shipment by Standard Mail
In most cases, samples may be shipped to the laboratory using standard postal services at ambient temperature. Many analytes exhibit high stability in the dried format, even at room temperature, as the drying process inactivates enzymes that could otherwise degrade proteins and other biomolecules. As a result, ambienttemperature shipment is often a robust and costefficient solution.
Capitainer® has designed a paperbased drying pouch to:
- Reduce waste and minimize carbon footprint
- Provide adequate ventilation for efficient drying
- Offer additional physical protection to the sample card during shipment
Considerations for Sensitive Analytes
If target analytes are sensitive to humidity and/or temperature, alternative packaging solutions should be considered. These may include:
- Aluminumcoated plastic bags
- Inclusion of desiccant pouches
The appropriate shipping and storage approach must be established and validated for each analyte. Stability assessment should always be included as part of method development and validation.
The paper ”Dried blood spots: Effects of less than optimal collection, shipping time, heat, and humidity” offers a comprehensive introduction to these factors.
Regulatory Classification and Labeling
Dried specimens are exempt from classification as dangerous goods and may therefore be shipped using standard postal services. According to the WHO Guidance on Regulations for the Transport of Infectious Substances, dried blood spots (DBS) are classified as exempt human specimens and are not subject to the requirements for transport of infectious substances (UN3373), provided there is a minimal likelihood that pathogens are present. [1]
This guidance is documented in the WHO publication “Guidance on regulations for the transport of infectious substances” and is reflected in multiple national regulations. For example, the U.S. Centers for Disease Control and Prevention (CDC) states that DBS specimens may be shipped by regular mail when triplepackaging requirements are met [2], which is ensured by the Capitainer® drying pouch design.
[2] Shipping Guidelines for Dried-Blood Spot Specimens (cdc.gov)
6) Post Analysis – Disposal and Biobanking
In conventional venous blood sampling, relatively large blood volumes are collected, while only a small fraction is typically used for analysis. The remaining blood is therefore discarded. Selfsampling with Capitainer® devices significantly reduces this waste by collecting only the volume required for analytical purposes. Disposal of used dried samples must always be performed in accordance with local regulations and institutional wastehandling procedures.
If samples are not discarded, they are often retained for biobanking. Venous samples are typically transferred to tubes and stored at –80 °C, which is associated with high costs, substantial energy consumption, and significant storagespace requirements. Microsampling cards provide an alternative approach, enabling biobanking in a more spaceefficient, costeffective, and energyconscious manner.
The paper ”Stability of Proteins in Dried Blood Spot Biobanks” discusses key aspects of longterm storage and stability of proteins in dried blood specimens and provides useful context for biobanking applications.
Capitainer® cards are composed predominantly of paper, with plastic limited to the microfluidic components and areas that come into direct contact with liquid. Capitainer continuously works to optimize product design and material selection in order to minimize environmental impact and reduce waste associated with sample collection and analysis.
We hope this information was helpful to you!
If you have additional questions we are happy to help you further through personal contact.
