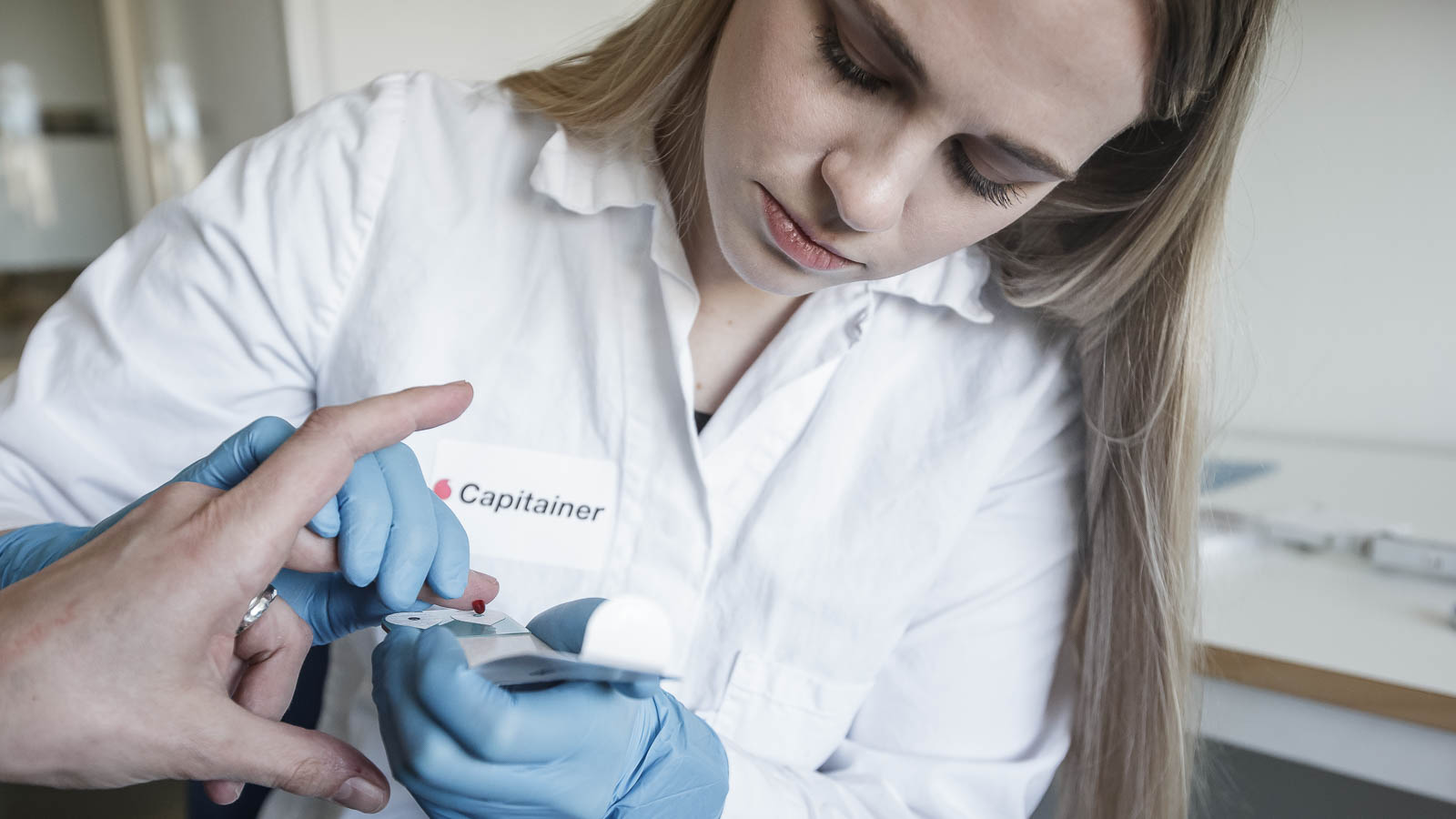
We have, as per April 30th, had our Novel micro sampling product, the Capitainer B, CE marked and registered with the Swedish Medical Product Agency (Läkemedelsverket).
The Capitainer B is registered as an IVD product according to the 98/97EC In vitro Diagnostic Medical Device Directive.
The novel Capitainer B product is a four channel blood sampling card that collects 13,5 microliter whole blood as dry blood spots, in each of four small cotton DBS paper pads.